Recently, Shenzhen Delica Medical Equipment Co., LTD. has confirmed that its latest TCD instrument, EMS-9D has successfully got FDA certificate.

With the official launch of EMS-9D , Delica TCD can provide CBFv for long-term monitoring. Meanwhile, this integral machine also supports ICM software which can collect multi-modality information from different devices. User can apply its preset calculation or even create their equations.
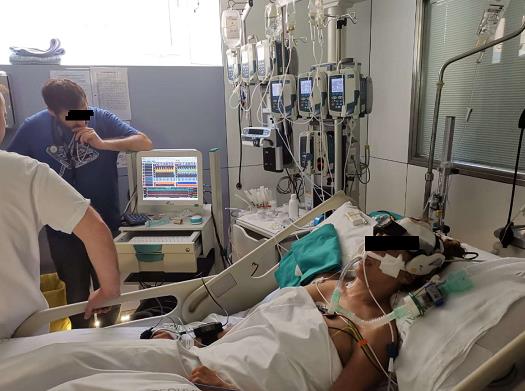
This module has been tested by the physic lab of Cambridge University for two years. it connecting with existing cNIBP manufactures like FMS/ CNSystems and other who provide analogue connectivity to the EMS 9 D. Also, it has a unique technology called Robotic Probe which makes TCD long-term monitoring in ICU possible . The integral ICM software also helps to collect multiple modalities from devices in ICU. It’s going to be an evolution to the whole TCD field.
 Delica Distributor Meeting in AmsterdamDelica Medical organized an international distributor meeting in Amsterdam, the Netherlands from April 2nd to 3rd. Nearly 28 participants from Europe and some other countries took part in this meeting.
Delica Distributor Meeting in AmsterdamDelica Medical organized an international distributor meeting in Amsterdam, the Netherlands from April 2nd to 3rd. Nearly 28 participants from Europe and some other countries took part in this meeting. Delica in Medica 2024From November 11 to 14, 2024 Dusseldorf International Medical and Medical Device Fair (MEDICA) was held in Dusseldorf Exhibition Center, Germany.
Delica in Medica 2024From November 11 to 14, 2024 Dusseldorf International Medical and Medical Device Fair (MEDICA) was held in Dusseldorf Exhibition Center, Germany. DELICA made a wonderful appearance in MEDICAFrom November 13 to 16, 2023 Dusseldorf International Medical and Medical Device Fair (MEDICA) was held in Dusseldorf Exhibition Center, Germany.
DELICA made a wonderful appearance in MEDICAFrom November 13 to 16, 2023 Dusseldorf International Medical and Medical Device Fair (MEDICA) was held in Dusseldorf Exhibition Center, Germany. DELICA made a wonderful appearance in the 87th CMEFFrom May 14 to 17, 2023, the 87th China International Medical Equipment Fair (CMEF) was grandly opened in Shanghai National Convention and Exhibition Center. Nearly 5,000 elite enterprises gathered in the event, covering more than 320,000 square meters, nearly 100 conferences and forums, hundreds of special activities, and tens of thousands of products. Industry celebrities and elite gathered in the scene, innovative technology and cutting-edge academic blend here, infinite business opportunitie
DELICA made a wonderful appearance in the 87th CMEFFrom May 14 to 17, 2023, the 87th China International Medical Equipment Fair (CMEF) was grandly opened in Shanghai National Convention and Exhibition Center. Nearly 5,000 elite enterprises gathered in the event, covering more than 320,000 square meters, nearly 100 conferences and forums, hundreds of special activities, and tens of thousands of products. Industry celebrities and elite gathered in the scene, innovative technology and cutting-edge academic blend here, infinite business opportunitie Delica Medical Workshop at Haga Teaching hospitalDelica Workshop at Haga Teaching hospital. Thank you Dr Ruud Keunen for the wonderful lectures!
Delica Medical Workshop at Haga Teaching hospitalDelica Workshop at Haga Teaching hospital. Thank you Dr Ruud Keunen for the wonderful lectures! Delica Medical Participated in CARNET 2022Shenzhen Delica Medical Equipment Co., Ltd. (Delica) Participated in the Cerebrolvascular Research Network (CARNet) meeting in Leicester, UK during 27 th – 29th October 2022.
Delica Medical Participated in CARNET 2022Shenzhen Delica Medical Equipment Co., Ltd. (Delica) Participated in the Cerebrolvascular Research Network (CARNet) meeting in Leicester, UK during 27 th – 29th October 2022. Delica Medical at European Stroke (ESOC) MunichShenzhen Delica Medical Equipment Co., Ltd. (Delica) Participated in the European Stroke (ESOC) 2023 Munich, a lot of interest in High Tech Robotic Probes on Delica TCD
Delica Medical at European Stroke (ESOC) MunichShenzhen Delica Medical Equipment Co., Ltd. (Delica) Participated in the European Stroke (ESOC) 2023 Munich, a lot of interest in High Tech Robotic Probes on Delica TCD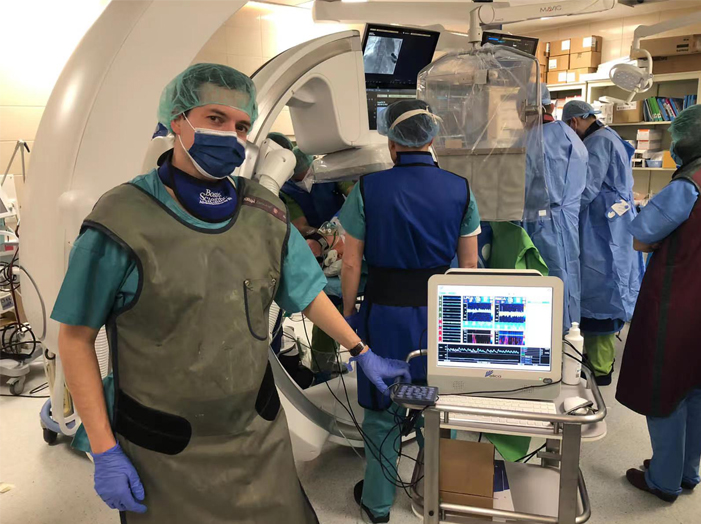 Robotic TCD monitoring during TAVI ProcedureTranscranial Doppler(TCD)is a useful diagnostic tool that can be used at bedside to evaluate patients with cerebrovascular disease. As we all know the clinical application from the AAN’s guideline such as Sickle cell screening
Robotic TCD monitoring during TAVI ProcedureTranscranial Doppler(TCD)is a useful diagnostic tool that can be used at bedside to evaluate patients with cerebrovascular disease. As we all know the clinical application from the AAN’s guideline such as Sickle cell screening The 23rd China International HI-TECH FAIRThe 23rd China International HI-TECH FAIR took place in Shenzhen on 27th-29th Dec, 2021 with the theme of advancing high-quality development and fostering a new development pattern.
The 23rd China International HI-TECH FAIRThe 23rd China International HI-TECH FAIR took place in Shenzhen on 27th-29th Dec, 2021 with the theme of advancing high-quality development and fostering a new development pattern. MEDICA 2021, Delica Medical showcases its innovative product .Delica Showcasing Their Latest Generation Product and Technology innovation.
MEDICA 2021, Delica Medical showcases its innovative product .Delica Showcasing Their Latest Generation Product and Technology innovation.